Chapter 4 Supplemental Problems The Structure Of The Atom
Chapter 4 Section 3. NCERT Class 9 Science.

Pearson Chap 4 Practice Name Date Class Atomic Structure Practice Problems In Your Notebook Solve The Following Problems Section 4 1 Defining The Course Hero
The structure of Al 3 Zr Formula unit Al 3 Zr Space group.

. The electrodes take on the charge of the battery terminal to which they are connected In a typical Cathode ray tube The. Matter and Change Chapter 4 5 The Structure of the AtomThe Structure of the Atom 1. Pick a past experiment.
Use the periodic table to complete the following table. Use the data in the table below to answer the questions that follow. T K Bansal for visually impaired students.
Chapter 4 - Structure of the Atom NCERT Class 9 Science Book Download and read NCERT Class 9 Science Books online. 2 Electrons revolve around the nucleus. It is a very important chapter and referring to.
Structure of the Atom STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by elizabethkinglol Terms in this set 21 alpha particle a particle with two. These books are the most up-to-date and the most authentic. Chapter 4 - The Structure of the Atom Chapter 4 Section 1.
I4m mm Cell contents. A subatomic particle in an atoms nucleus that has a positive charge of 1 neutron a neutral subatomic particle in an atoms nucleus that has a mass nearly equal to that of a proton atomic. This Chapter is planned organized and described by Dr.
Matter is composed of extremely small particles called atoms. 1 All matter is composed of extremely small individual indivisible indestructible particles called atoms gives Democritus credit 2 Atoms of a given element are identical in size mass. Students who are studying in Class 9 and use the NCERT Textbook to study Science will have come across Chapter 4 Structure of the Atom.
Early Ideas about Matter. Structure of the Atom Pieces of evidence that scientists had in 1900 to indicate that the atom was not a fundamental unit. 3 Certain special orbits known as discrete orbits of electrons are allowed.
Chapter 4 Section 2. Chapter 4 - The Structure of the Atom Describe one of the experiments that contributed to the understanding of the atom and its structure. 1 There seemed to be too many kinds of atoms.
Chapter 4 STRUCTURE OF THE ATOM. NCERT Solutions for Class 9 Science Chapter 4 Structure Of The Atom. An atomic mass unit amu is equal to 1-12th the mass of a------atom.
Terms in this set 43 Daltons Atomic Theory. Atoms could not be created destroyed or further divided. Atoms of a given element are.
Atoms are indivisible and indestructible. Bohrs model of the atom 1 Atom has nucleus in the centre. I the mass of the atom is assumed to be uniformly distributed over the atom ii the positive charge is assumed to be uniformly distributed over the atom iii the electrons are uniformly.
Different kinds of atoms have different sizes and shapes that determine the properties of matter. Supplemental Problems for Chapter 4 1. Chapter 4 Structure of the atom of NCERT Class 9 Science is categorized under Unit I Matter Its nature and.

Class 9 Archives Page 7 Of 9 Esaral

Modeling Nanoscale Cellular Structures Using Molecular Dynamics Sciencedirect

Extra Questions For Cbse Class 9 Science Term 2 Exam 2022 With Answers Best For Quick Revision

Section 4 1 Studying Atoms Barrington High School

Data Structures Algorithms In Dart Raywenderlich Com

Atomic Structure Lesson Plans Activities Videos Lessons Study Com

Periodic Table Wikipedia

Atomic Nucleus Structure Composition What Is Atomic Nucleus Video Lesson Transcript Study Com
Structure Of The Atom M System Springerlink

Vitamin B12 Wikipedia

5 Molecular Physics Atomic Molecular And Optical Physics The National Academies Press
Chapter 4 The Structure Of The Atom Mr Saint S Science Website
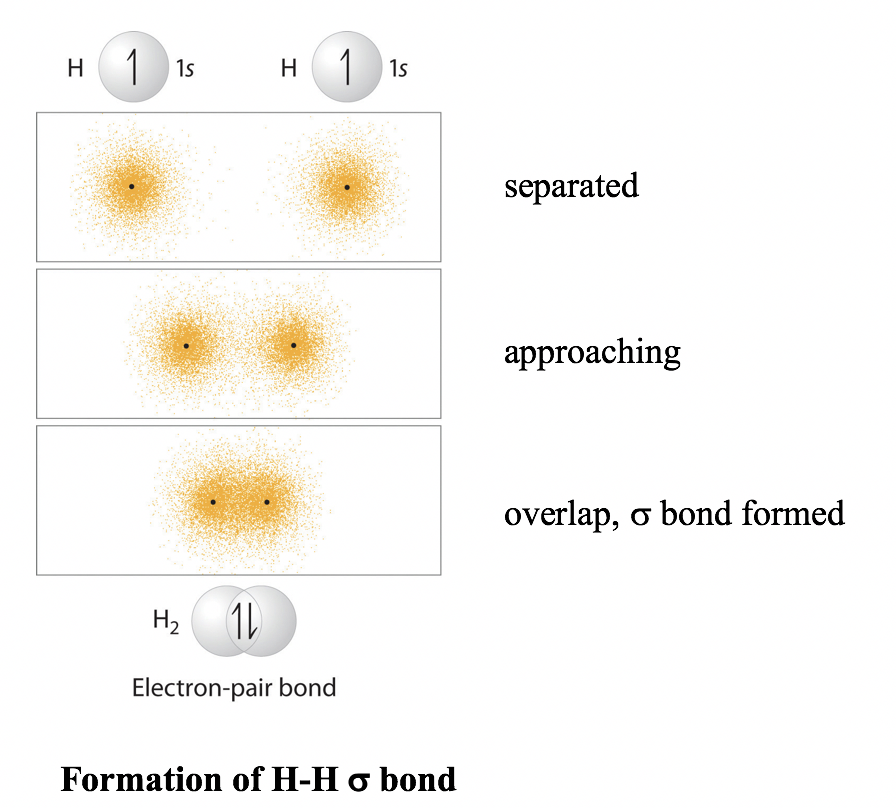
1 6 Valence Bond Theory And Hybridization Organic Chemistry I
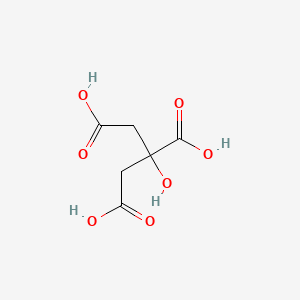
Citric Acid C6h8o7 Pubchem

Chemistry Zimsec Chapter 4 Chemical Bonding
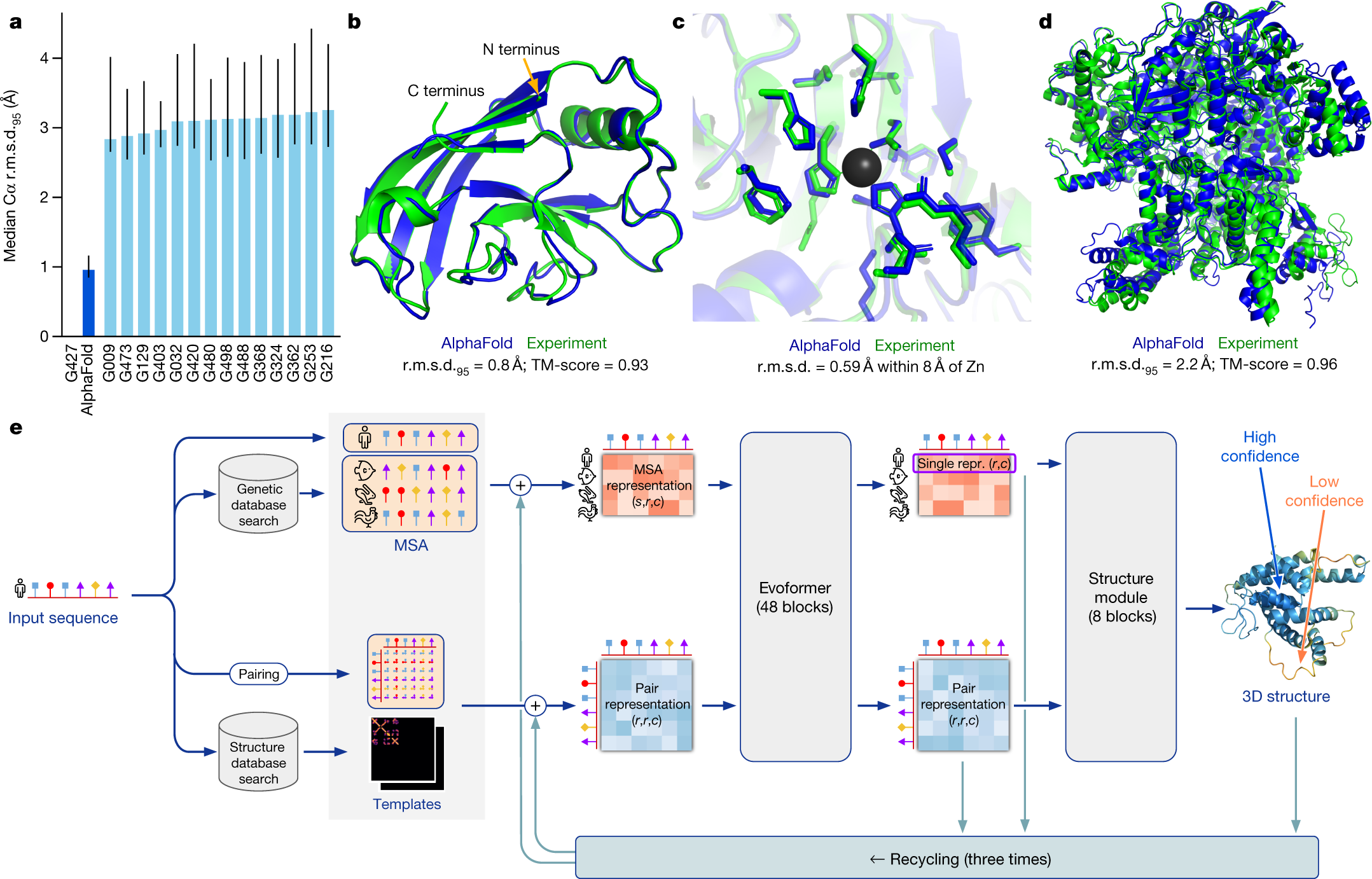
Highly Accurate Protein Structure Prediction With Alphafold Nature
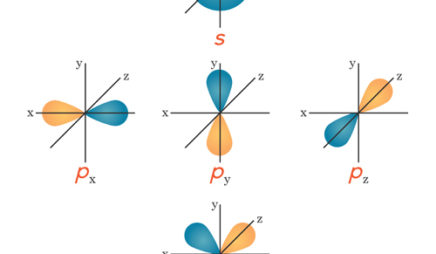
Organizing Atoms And Electrons The Periodic Table Annenberg Learner